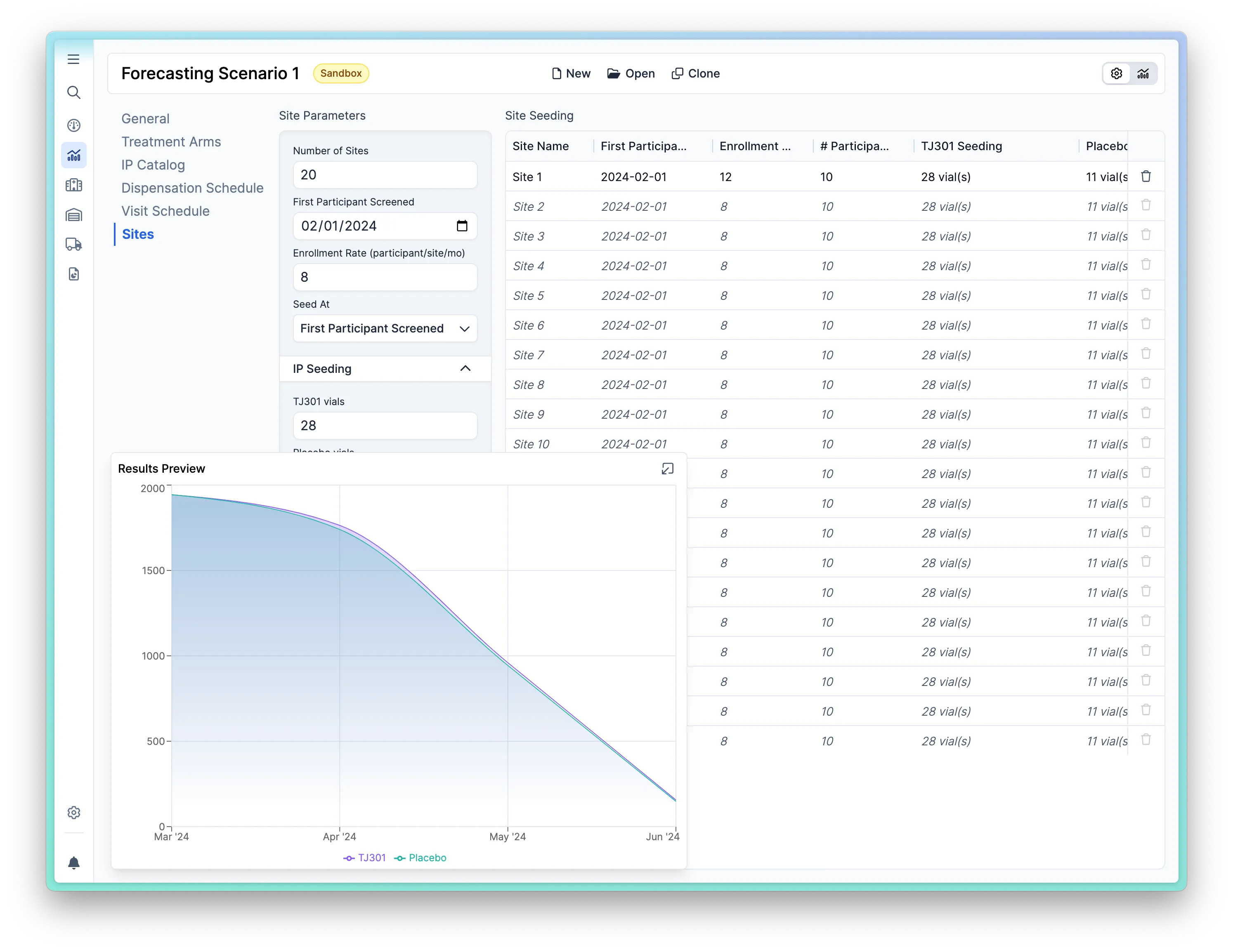
Using parameters such as enrollment rates and dates, instantly calculate study drug demand, shipping, and manufacturing dates.
Quickly forecast supply needs for your study using our AI-driven study builder. Test multiple scenarios: “what happens if we increase enrollment from X to Y?”
Automatically populate live actuals from Bluefin RTSM as your study progresses. Narrow your future uncertainty bands without having to manually import or stitch together datasets.
Allows you to forecast at the site, country, or study level. Choose to set up sites individually or handle them in regional groups. Kit materials or opt to forecast them individually.
Build studies in minutes, not weeks, using Bluefin's no-code configuration tool. Get started quickly by simply uploading a protocol and let Bluefin take care of the initial configuration for you.
Studies move quickly and amendments need to happen fast. Amend your protocols with minimal downtime. Get approvals from sites and consent from participants. Bluefin maintains a full audit trail across all protocol versions. And with no expensive change orders, ever.
Track item histories from inception through end-of-study and destruction. Inventory, shipment, cold chain, dispensing, and accountability - full visibility without blind spots.
Simple enough for Phase I studies yet powerful enough for global, Phase III pivotal trials. Whether you need complex orchestration or just want to digitize participant randomization, investigational product and accountability logs, Bluefin is the ideal choice.
Bluefin uses modern SaaS best practices, such as continuous integration and deployment, to keep your company's data secure. And using Bluefin will help your studies comply with 21 CFR Part 11 by logging all user actions with compliant electronic signatures.
Bluefin’s reporting engine allows users to generate their own reports using any data they have permission to access. Pivot, graph, save, and export data as you please. Pin your reports to your dashboard for quick reference.
We believe you, not us, own your data. That’s why our API is complete and documented - allowing you to push data into our system, or pull it out. Integrate with anybody. In fact, Bluefin’s user interface is built using our own API, so we know it works.